TUV

CCC

CE

UL


ISO9001:2008

ISO14001:2004

ROHS
Quality
Quality
Summary:
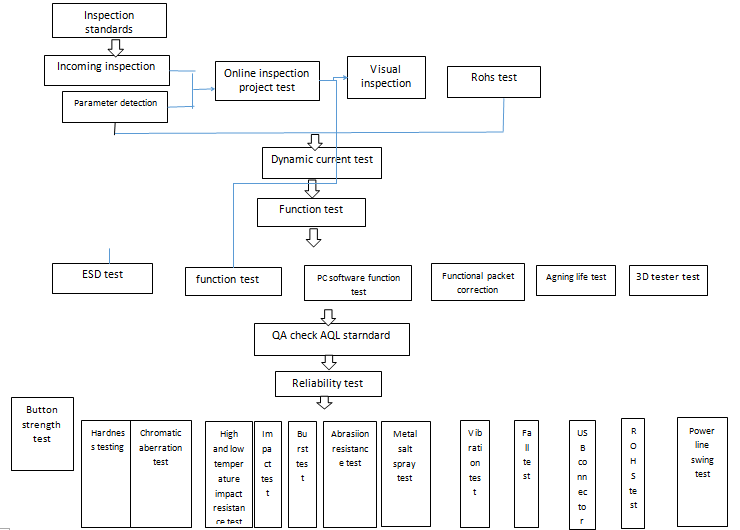
The quality department of Hengyong Group is directly under the management. Quality is the lifeblood of the company and the life of the product. In 2003, it passed the ISO9001 certification of quality system. In 2006, it passed the ISO14001 certification of environmental protection system. In 2018, it officially passed the ISO13485:2016 quality system certification for exclusive medical products, ensuring full product monitoring and compliance with international and domestic laws and regulations. The company established a reliability laboratory in 1996 to ensure the authenticity of the entire process of product verification! Includes high and low temperature humidity test, wear resistant and resistant test, hardness test, impact test, salt spray test, drop test, vibration test, socket plug test, power wire rocking test, crack test, waterproof test, life test, 3D Three-dimensional test, color difference test, thickness test, ICP optocoupler ROHS test, aging workshop test, ESD test, network communication test and so on. Let quality have a basis!
-

- Reliability lab
-

- RoHs lab
-

- Aging experiment
-

- 3D test lab
-

- RF test lab
-

- Color wheel test lab
-

- Finished text room
-

- Visual inspection room
-

- QA room
QC support:
Establish a quality assurance system, monitor all processes, use SPC data statistical analysis to improve, provide correction and track implementation. Regularly provide training and assessment for employees.
Quality department:
69 person
Medical product testing process links:
In 2002, the company passed ISO9001:2008 quality management system, ISO14001:2004 environmental management system certification, and established APS environmental pollution prevention system. In 2018, it passed ISO13485:2016 exclusive medical product quality management system. We strictly follow the ISO process to ensure that every aspect of our products meets our customers' requirements and social laws and regulations.